There are many industries where ‘Data Integrity’ is of utmost importance, ranging from Contract Labs and Food & Agriculture to Pharmaceutical and Biotechnology industries.
They all want to show that they follow systematic processes that deliver reliable, repeatable and traceable results. Some of these labs will follow strict industry regulations or guidelines, such as GxP, 21 CFR part 11 and Annex 11; or they may have internally generated processes to prove that their results can be relied upon.
Challenges of regulating EDS
It has been, and continues to be, a challenge to incorporate the SEM-EDS technique into a regulated process, mainly due to the non-routine nature of most analysis in the SEM. There are a number of users that follow IQ/OQ/PQ processes, which also have ramifications on the SEM-EDS installation and operation.
Let’s look at the example of EDS analysis working under 21 CFR part 11 (which covers the use of electronic records in the regulated pharmaceutical, biotechnology and medical device industries). Because of the complexity, there is currently no EDS software platform that conforms totally with these guidelines. This is mainly due to the size of the market and the significant investment required to deliver a fully compliant product. An EDS project can contain hundreds of files that are accessed on a continuous basis during an analysis. Unlike acquiring an electron image, which will only have grey level information in each pixel, an X-ray SmartMap will have a full set of elemental data in each pixel. That data can then be processed and presented in different ways.
Because of the non-conformity of the EDS software, the burden of achieving conformity for an SEM-EDS system ultimately falls to the user. They must evaluate all their equipment and perform a risk assessment to identify all the areas that do not conform.
There will often be a collaboration between the User, Quality Manager and the IT Manager. They will put in place a Standard Operating Procedure to ensure that, although the equipment doesn’t conform in isolation, the whole analysis process does.
Although Oxford Instruments were not able to supply a fully compliant EDS software platform, we were asked if there was anything we could do to make the process of conformity easier for users. So, we formed a user panel and together we generated and prioritised a list of the most important features needed to improve the conformity process:
• User Login
• Audit Trail
• Audit Trail Viewer (capability to Archive and Backup the database)
• Data Integrity - Prevent modification or deletion of data
These users also requested that the EDS software must consider the analysis process itself, and not:
• Make the process unnecessarily complicated
• unnecessarily long
• Be so inflexible that it inhibits getting the right answer
AZtecPharma
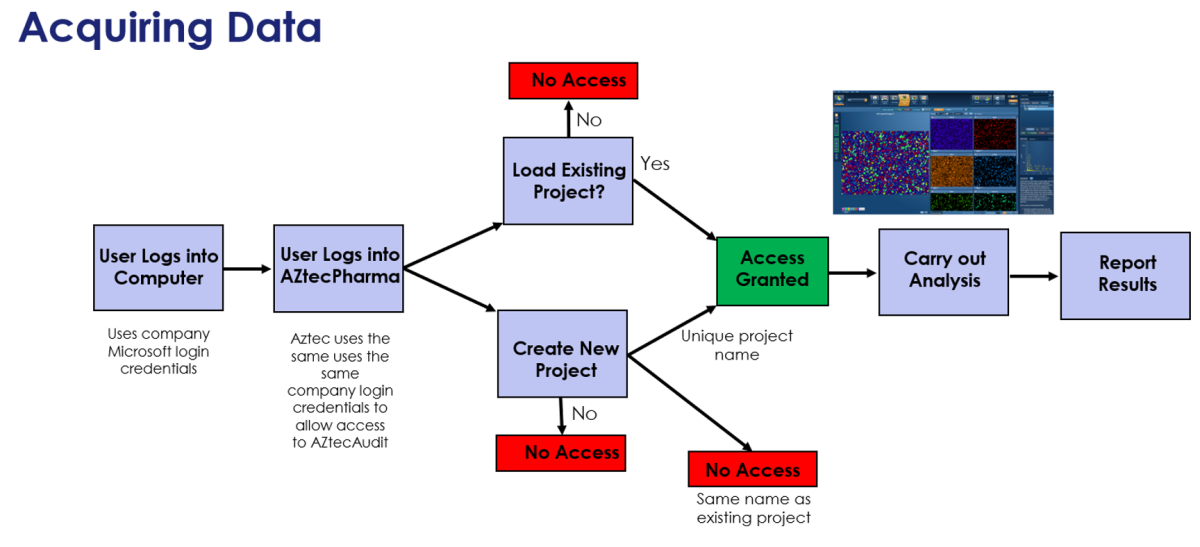
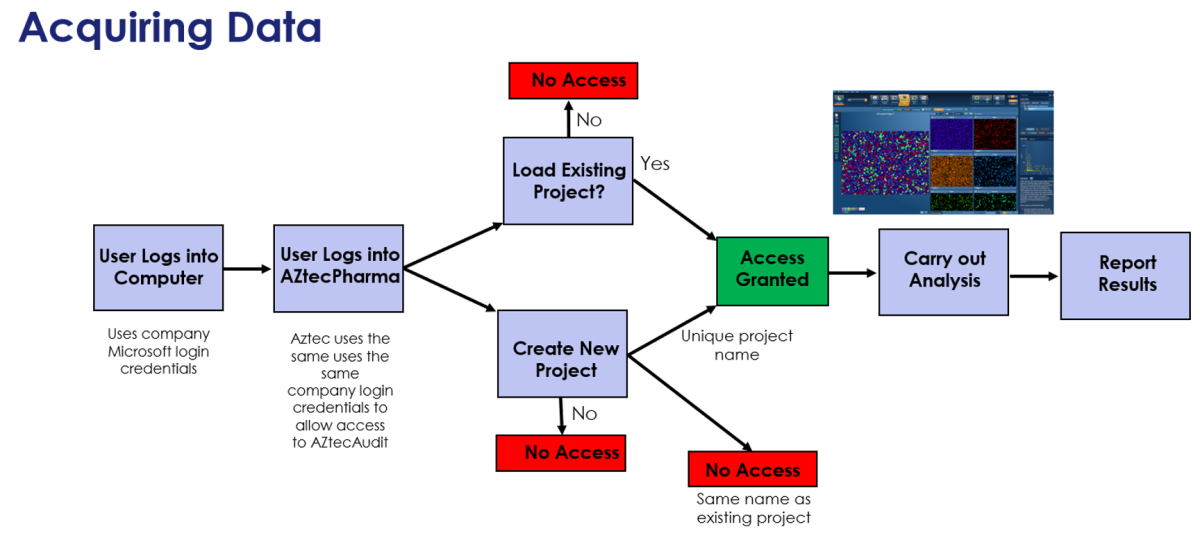
The first step to addressing these requirements was to create a dedicated version of AZtecLive called AZtecPharma. This new platform has the same look and feel as AZtecLive, but with only EDS capabilities. To satisfy the first requirement, AZtecPharma has a “User Login” screen to gain access. It uses ‘Windows Credentials’, which means that the local IT department can control user access and password protocols.
AuditTrail
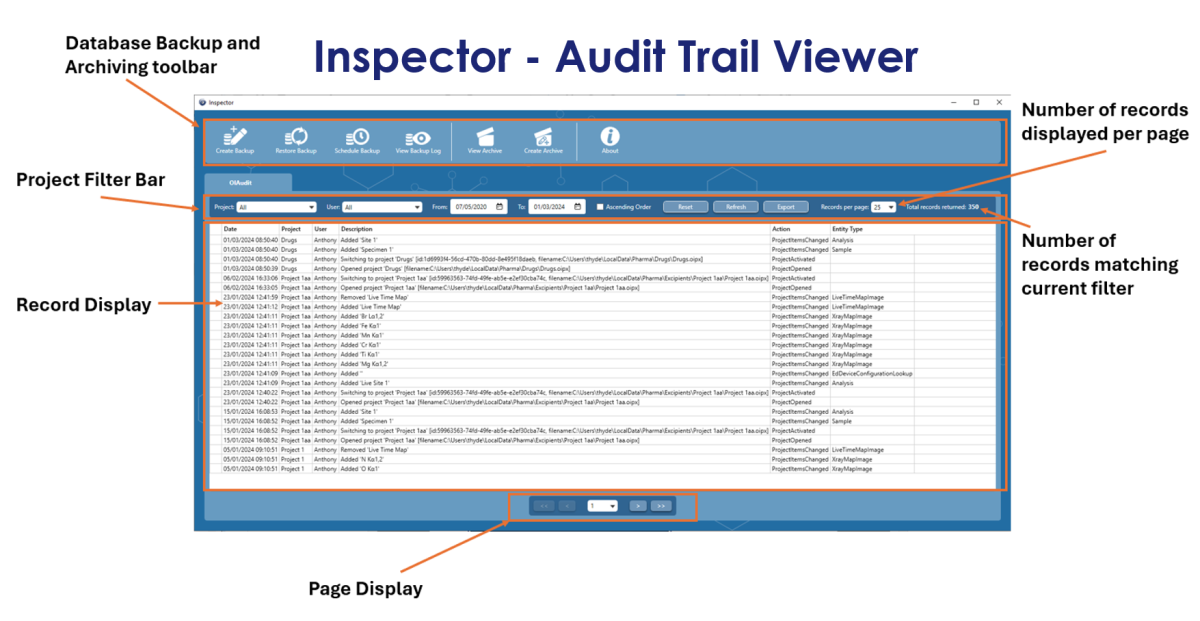
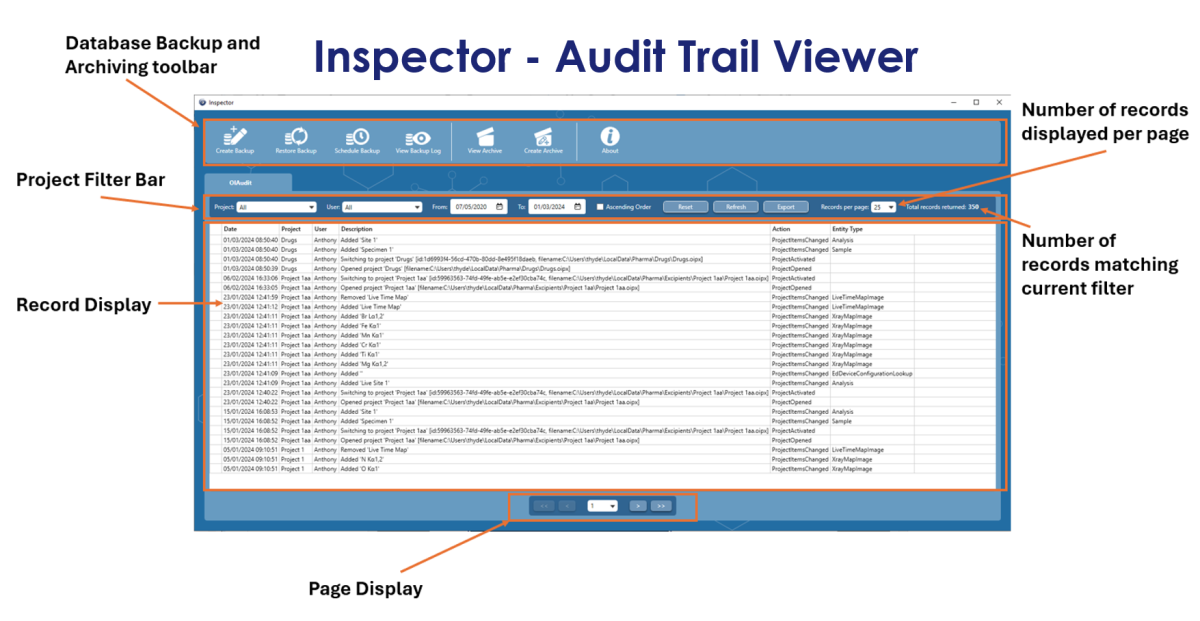
User login functionality is crucial to the whole process, because knowing who is using the software means that we can record and stamp all major data interactions with the username, date and time, which in turn generates an “Audit Trail”. Obviously, for this to work, the user created SOP needs to state that all users must not share their login details and not leave the system unattended and logged in. Then, we have the requirement to view this audit trail. The most straightforward way of achieving this was to create a dedicated viewer, which we called “Inspector.” This program not only enables the user to view the records, but also allows the user to filter records based on the username, project name, time and date. It’s not enough to be able to view the audit trail, because users often have strict backup and archiving procedures. So, the Inspector program enables the user to setup regular Backups, as well as Archiving portions or all of the database, based on date. The archived database can then be viewed at a later date, within the same Inspector program.
Data integrity
When it comes to “Data Integrity”, AZtecLive and AZtecPharma already have built in security. They both create 3 data entities, none of which can be modified or deleted:
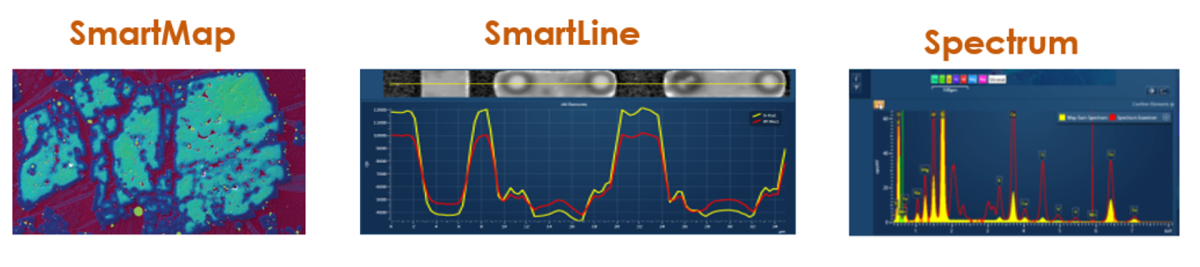
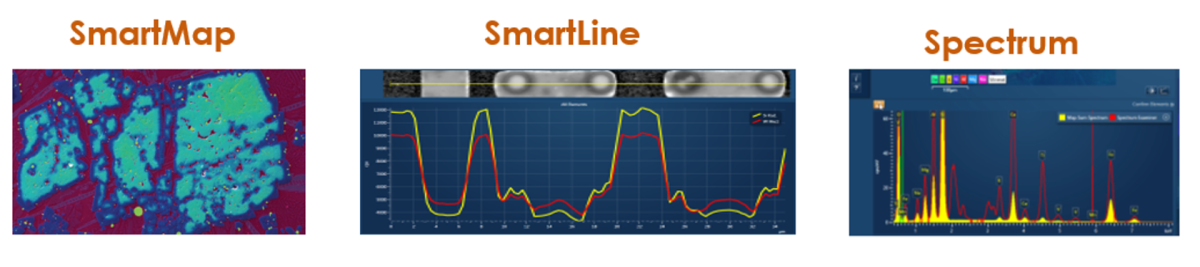
1. SmartMap – Contains spectral data from an area of the sample, where each pixel contains a full set of X-ray data.
2. SmartLine – Contains spectral data from a line on the sample, where each pixel contains a full set of x-ray data
3. Spectrum – Contains spectral data from a point on the sample
In addition, the option to delete data within AZtecPharma has been removed.
Usability
Regarding the concerns over “Usability”, once logged into AZtecPharma, the analysis process is the same as with AZtecLive. The only differences are that they must login, then they must either load or create a unique project.
In summary
To summarise, we can say that, although the regulated market is not large enough to warrant the cost of developing a fully compliant EDS software platform, there are steps that EDS manufacturers can take to make the process of becoming compliant much easier for the user.