I joined Oxford Instruments in June 2022 after a short stint in academia post PhD. In the two years I spent as a post-doc, most of my research focused on building instrumentation to enable hyperpolarised nuclear magnetic resonance (NMR) applications. These applications aim to boost the signal obtained from an NMR experiment by 5 or 6 orders of magnitude and can be used in cancer metabolomics1 and imaging2. In this blog I will introduce the topic of hyperpolarisation and show how it has been used in benchtop NMR.
Nuclear Magnetic Resonance (NMR) is a powerful non-invasive, non-destructive technique that is employed in myriad ways across academia and industry in areas such as molecular structure determination, quantification of active pharmaceutical ingredient (API), and quality control. One thing that limits NMR is sensitivity, even the most powerful superconducting magnets produce a very small net magnetic alignment of nuclei, and it is this net alignment that is responsible for the signal. One way that the field of NMR quantify sensitivity is by using the signal-to-noise ratio (SNR), and as such, there are two main avenues for improving this number. The first is to reduce the noise, which is done primarily by cooling the electronics of the probe to cryogenic temperatures. This reduces the thermal noise and can improve the SNR by up to a factor of 5. The second way to improve the SNR is to increase the signal, and this means that the net alignment must be increased in some way. Once the net alignment, or polarisation, of the system is above ~0.1% it is referred to in NMR as ‘hyperpolarised’.
Hyperpolarisation can be accomplished in several ways3. The way I am most familiar with is called parahydrogen induced polarisation (PHIP). Parahydrogen is a spin isomer of hydrogen that can be obtained by cooling hydrogen in the presence of a suitable catalyst. There are two main forms of PHIP typically reported in literature, these are hydrogenative and non-hydrogenative. Hydrogenative PHIP, as you may have guessed, involves the irreversible addition of hydrogen to a substrate and non-hydrogenative PHIP typically involves a hydrogen molecule reversibly binding to a metal-based catalyst at the same time as a target molecule and transferring the polarisation via the metal catalyst.
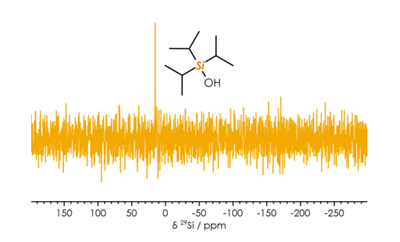
Figure 1: Single scan of 30 mM of trimethylsilanol using SABRE-Relay.
The latter type of PHIP was demonstrated recently on X-Pulse during a visit from the Duckett Group from the University of York. We took advantage of the Signal Amplification By Reversible Exchange (SABRE)4 effect to hyperpolarise protons, carbon-13 and fluorine-19 observing signal enhancements of well over 1000x for the carbon and fluorine nuclei. The result I found most impressive was the SABRE-Relay effect we observed on silicon-29, a notoriously difficult nuclei to measure using benchtop NMR.

Figure 2: Researchers from the University of York and Robin Blagg from Oxford Instruments performing the experiments.
Silicon samples, even at high concentrations, can take hours of measurement time to observe, however, when using this hyperpolarisation method on the X-Pulse a 30 mmol sample of silicon was observed in just one scan!
In conclusion, hyperpolarisation can be an effective way to overcome the sensitivity limitations in NMR, providing you have the suitable equipment to implement it. The flexibility and portability of benchtop NMR mean it is ideally suited to performing hyperpolarisation experiments. For example, the liquid transfer lines can be shorter and it’s easier to insert the sample in comparison to high-field instruments. As X-Pulse is a tuneable broadband instrument, it can also detect the hyperpolarisation of more exotic nuclei, like silicon-29.
To find out more about X-Pulse Broadband Benchtop NMR click here.
References:
- V. Ribay; C. Praud; M. P. Letertre; J.-N. Dumez & P. Giraudeau, Current Opinion in Chemical Biology, 2023, 74, 102307.
- P. E. Z. Larson; J. M. L. Bernard; J. A. Bankson; N. Bøgh; R. A. Bok; A. P. Chen; C. H. Cunningham; J. W. Gordon; J. Hövener; C. Laustsen; D. Mayer; M. A. McLean; F. Schilling; J. B. Slater; J. Vanderheyden; C. von Morze; D. B. Vigneron & D. Xu, Magnetic Resonance in Medicine, 2024, 91, 2204-2228.
- J. Eills; D. Budker; S. Cavagnero; E. Y. Chekmenev; S. J. Elliott; S. Jannin; A. Lesage; J. Matysik; T. Meersmann; T. Prisner; J. A. Reimer; H. Yang & I. V. Koptyug, ACS, 2023, 123, 1417-1551.
- R. W. Adams; J. A. Aguilar; K. D. Atkinson; M. J. Cowley; P. I. P. Elliott; S. B. Duckett; G. G. R. Green; I. G. Khazal; J. López-Serrano & D. C. Williamson, Science, 2009, 323, 1708-1711



